Mineral acids were some of the most frequently used substances in Newton's chymistry. These included "oyl of vitriol" (sulfuric acid), "aqua fortis" (nitric acid), and "spirit of salt" (hydrochloric acid), in addition to various combinations of these such as "aqua regia" (nitric acid & hydrochloric acid). Drawing from the works on "strong waters" of Islamic alchemists such as Jabir ibn Hayyan (c. ninth-tenth century) and Abu Bakr Muhammad ibn Zakariya' al-Razi (c. 865-925), medieval alchemists and physicians were the first to produce concentrated acids from the distillation of minerals (Robert Multhauf, The Relationship Between Technology and Natural Philosophy, ca. 1250-1650, as Illustrated by Technology of the Mineral Acids (Ph.D. Dissertation, University of California, 1953), pp. 140-141). Newton describes these acids and the method for making them in the manuscript Oxford University, Bodleian Library Don b. 15.
Don b. 15 pp.4r & 7r:
"Oyle of Vitrioll is acid destild from vitrioll first calcined to whitenesse for feare of boyling over when it <illeg.> . The fumes are white but sattle into this reddish liquor."
Newton has another description of the process in the same manuscript on p.8v:
"Spt of Vitrioll & Oyle. Deflegm ye vitrioll &c a <illeg> yt circulatory fire till after melting it coagulate into a grayish lump wch is done in 2 howers. A glass retort half filld wth this poudered, & urged into a large receiver till black veines begin to trickle downe. Then change ye receiver but lute it not on. A pound yeilds 9 or 10℥cs of transparent spirit, 1 1/2
℥ of black oyle, & ye remaining colcothar (caput mortuum) conteins a fixed salt of
♀. The spt & oyle differ but in their flegm: ffor a drachm of spt dropt into common water
℥i, & filtered makes ye spt."
The English chymist and physician John French (1616-1657) described a similar method for the production of oil of vitriol, and he also included a picture of the apparatus used for distilling various mineral acids.
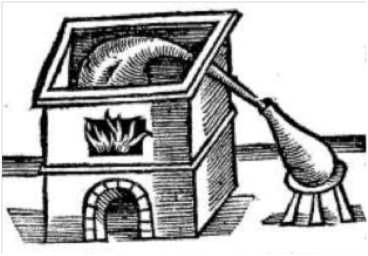
French describes the process as follows:
"Take of Hungarian, or the best English Vitrial, as much as you please, let it be melted in an earthen vessell glazed, with a soft fire, that all the moisture may exhale, continually stirring of it, untill it be brought into a yellow powder, which must be put into a glasse Retort well luted, or an earthen Retort that will endure the fire: Fit a large Receiver to the Retort and close the joints wel together; then give it fire by degrees till the second day, then make the strongest heat you can til the Receiver which before was dark with fumes be clear again; let the Liquor that is distilled off be put into a little Retort, and the flegm be drawn off in sand, so will the oil be rectified, which is most strong and ponderous, and must be kept by it self."
In our reproduction of Newton's method for making the oil of vitriol, we constructed a temporary furnace using a steel tabletop barbecue surrounded by insulating firebrick. For a fuel source we used 100% natural hardwood charcoal. We used a specially made Pyrex retort and passed the condensing arm through a bucket of ice into a receiving flask. As per Newton's instructions, we did not seal the receiving flask to the retort.
We "dephlegmed" our vitriol (FeSO4·7H2O) in a glass crystallizing dish on a hotplate until the material ceased evolving steam, had lost its green and blue color, and appeared a greyish yellow. After allowing the material to cool, we placed it in the glass retort and began heating in the fire.


Shortly after the temperature reached 400° C as measured by a thermocouple probe rated to 1000° C the color of the ferrous sulphate began to change to red. This is the temperature at which ferrous sulphate decomposes.

We immediately began to evolve a clear, acidic vapor, which we measured using standard pH paper (0-14). The approximate value of the pH of the vapor was zero.

After proceeding with the distillation for approximately 4 hours at increased temperatures (the highest was near 1000°C), the evolution of vapor slowed and we ceased to collect acid. The product obtained was a clear, highly acid liquid with a pungent (and painful) odor.

Some water from our condenser seeped into the receiving flask and diluted our acid, so we concentrated it using a traditional alchemical "Alembic." After removing the excess water from the acid, the product was more viscous and had a measured pH of zero.

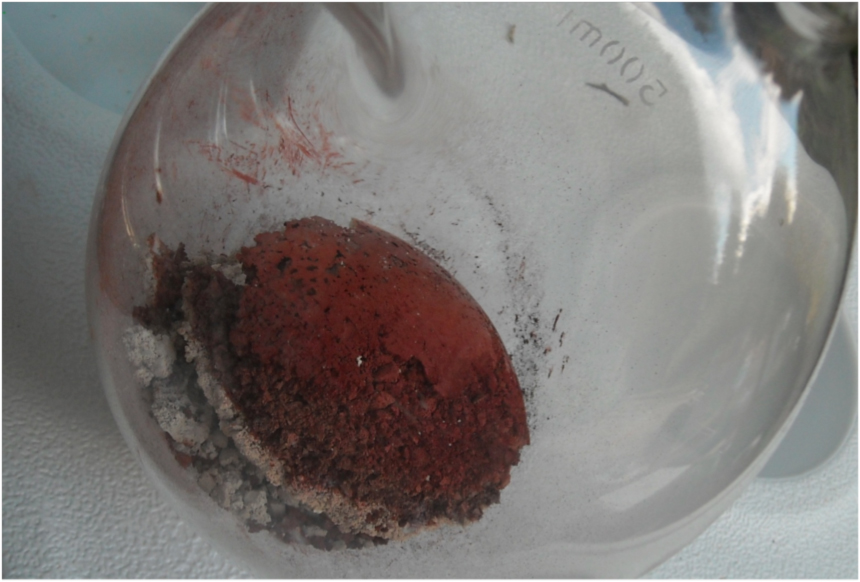
After the glass retort had cooled, we observed that the fire had been hot enough to deform the Pyrex retort. The ferrous sulphate touching the bottom of the retort had turned bright red, but the other material was a greyish white.

Newton describes the production of aqua fortis in Don b. 15, p.1r:
"Aqua fortis is acid is distilled from onete of Salt peeter well mixed wth two
ts of Vitriol well calcined to white. The fumes are red but settle into this cleer liquor. It differs very little from spirit of Niter, for ye Vitriol yeilds little or nothing but is only added to keep ye Niter from <flux>ing; for Salt & Salt peeter yeild noe spirit while they are in flux. It dissolves all mettalls <bu>t Gold. It may bee also drawne from a soluti<on> of one part of Salt peeter mixed wth one part of Oyle of Vitriol <illeg.> the caput mortuum will afford a white salt &c."
John French has a similar description in his 1653 The Art of Distillation:
"Take of vitriol calcined two parts and of nitre one part. Grind and mix them well together and put them into a glass retort coated or earthen retort that will endure the fire. Set them into the furnace in an open fire and then, having fitted a large receiver, distill it by degrees the space of 24 hours. Then rectify the water or spirit in sand."
We began our distillation of aqua fortis with a similar apparatus as that used in our distillation of sulfuric acid. The furnace was practically identical (made from the tabletop barbecue and the firebrick, using natural charcoal as fuel), but instead of the glass retort and the ice condenser we used a 500mL roundbottom flask, a distilling adapter, a 400mm West condenser, and a large Erlenmeyer as a receiving flask. We cycled water through the condenser for the duration of the experiment.

As per Newton's description, we used two parts of calcined ferrous sulfate and one part potassium nitrate. We began heating the vessel and shortly after the temperature had reached approximately 500°C, we observed a red gas forming in the round bottom and the distillation adapter.

This gas was very likely NO2. The gas filled the whole apparatus and some of the material began to assume liquid form (presumably as the gas combined with water in the air).


We continued the distillation for approximately four hours, until it appeared that gas was no longer evolving. We collected the liquid acid in several vials but allowed the gas remaining in the receiving flask to sit overnight. Both the liquid and the vapor had a pH of zero (as measured with 0-14 pH paper).


Newton describes the production of the spirit of salt (hydrochloric acid) in Don b. 15, p.8v, as follows: "Spirit of Salt. Common salt, beat fine in 1 part brick-dust or potters earth not over dryed & pouder 5 parts : urge by a graduall fire out of a glass retort filld full into a large receiver till you feel the receiver cold & one pound will yeild nine or 10 ounces."
John French describes this process in much the same way: "Take one part of salt and three parts of powder of bricks or tiles, mix them together, and put them into a retort either of glass or earth, to which put fire as before. After this manner you may make oil or spirit of nitre, salt gem, alum. Note that these salts must first be calcined which is done by exhaling their phlegm."
Newton's description of the ratio of fuller's earth to salt was difficult to understand, so we chose instead to use French's ratio of three parts fuller's earth (cosmetic clay) to one part sea salt (purchased at a local health-food store). We used the same apparatus as the above aqua fortis distillation, but tried for a higher temperature as sodium chloride decomposes at 801°C.

By stacking our firebricks higher and using a larger amount of coal we were able to achieve higher temperatures, reaching and perhaps surpassing 1000°C according to our thermocouple (only rated to 1000°C). The distillation yielded a white, pungent gas and a clear, acidic liquid. We continued the distillation until liquid and gas ceased to evolve, but attempted to distill more product by making the fire very hot.

During the distillation the glass appeared to be warping from the heat. After the distillation was finished and the glass had cooled, we observed that the Pyrex round bottom flask had been significantly melted by the fire.

